Abstract
Introduction
Anemia is the most common blood disorder which affects billions of people, especially young adults and women. However, currently available treatments of anemia are with limitations and adverse effects. Therefore, natural resources have been receiving considerable attention as complementary or alternative hematinic agents in recent years. In this regard, olive leaf extract (OLE) is rich in bioactive phenolic compounds and has been reported to have anti-inflammatory, antioxidant and neuroprotective effects (Vogel et al., 2015). Our previous study showed treatment with water extract of olive leaf (WOL) for 12 days could induce erythroid differentiation in human hematopoietic stem cells (hHSCs) and showed potential in oxygen and iron homeostasis, haem metabolism, and hemoglobin (Hb) biosynthesis (Kondo et al., 2021). In the present study, we aimed to investigate the therapeutic effects of WOL on the phenylhydrazine (PHZ)-induced mice model of anemia and explore its underlying molecular mechanism.
Methods
Total 63 male ICR mice (four weeks old) were randomly divided into three groups: control, PHZ and WOL groups. Mice in the WOL group were orally administered with 150 mg/kg body weight of WOL (diluted by saline) every day, while mice in the other two groups received saline. After two weeks of WOL pretreatment, PHZ was injected intraperitoneally (60 mg/kg body weight) to PHZ and WOL groups. The mice were dissected on day0 (before PHZ injection), day1, day3, day5, and day7 after PHZ injection. We collected blood for hematologic tests during dissection and liver, kidney, intestine, bone marrow, and spleen samples. We next checked the iron homeostasis-related gene expressions by real-time PCR: hepcidin (Hamp) in liver, ferroportin (Fpn) in spleen and intestine.
Results and Discussion
After anemia was induced, WOL group showed a significant increase in the number of reticulocytes, the erythroid progenitors, compared to that of in PHZ group on Day 5. Simultaneously, plasma iron level on Day 5 and Hb level on Day7 was significantly decreased in the WOL group compared to the PHZ group. These results suggest that during anemia, the WOL group had rapid erythropoiesis, which in turn caused a rapid consumption of Hb and iron.
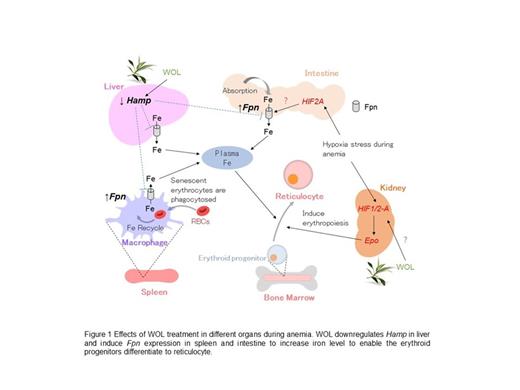
Additionally, mRNA expression of Hamp in liver was significantly decreased on Day 5, whereas Fpn expressions in spleen and intestine were significantly increased on Day 5 and Day 7 in the WOL group compared to the PHZ group.
Hamp regulates plasma iron concentrations as well as systemic iron metabolism by interacting with its receptor Fpn, a transmembrane iron-exporter. In intestine, Fpn controls iron absorption from food intake, while in spleen, Fpn regulates phagocytosis of senescent erythrocytes by the macrophages. Decreased Hamp expression and increased Fpn expression by WOL treatment suggest increased levels of absorbed and recycled iron to meet the demand for erythropoiesis. Altogether, our findings indicate that WOL could regulate iron homeostasis in the early stage of anemia to promote the differentiation of erythroid progenitors.
Conclusion
Our study suggests that WOL treatment downregulates Hamp expression in liver and increased Fpn expressions in the intestine and spleen, leading to increased absorbed iron in the intestine and recycled iron in the spleen (Figure 1). Therefore, WOL may have therapeutic potential in anemia through regulating iron homeostasis and promoting erythroid differentiation. However, further studies are required to confirm these findings and to elucidate the molecular mechanisms.
Our previous study showed that 12-day treatment of hHSCs with WOL could upregulate the expression of hypoxia-inducible factor 1-a (HIF1A) (Kondo et al., 2021), a key modulator of the transcriptional response to hypoxia and has been reported to regulate erythropoietin (Epo) production, which plays an important role in erythropoiesis. So, we hypothesize that WOL treatment would activate HIF1A in anemia-induced mice model, thus regulating Epo production to accelerate erythroid maturation. HIF2A is also reported to regulate Epo in kidney and Fpn in intestine to regulate erythropoiesis (Andrew J., et al., 2019).
Therefore, we will investigate Epo expression in kidney, Hif1a and Hif2a expressions in kidney and intestine, and transferrin receptor (Tfrc, erythroid marker) in bone marrow. All gene expressions will be confirmed in protein levels also.
Suidasari: Nutrition Act Co. Ltd.: Current Employment. Yokozawa: Nutrition Act Co. Ltd.: Current Employment. Yamauchi: Nutrition Act Co. Ltd.: Current Employment.